If you find this useful, please leave a comment at the end of the page.
Link to Section A.
Link to Section B. Questions 16 to 19.
20
This question is about aromatic compounds containing the –COOH and –OH functional groups.
(a)
Salicylic acid, shown below, is used in the manufacture of some important medicines.
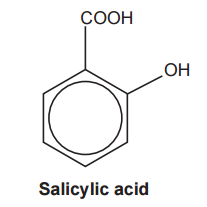
Complete the flowchart for reactions of salicylic acid, by adding the organic products in each box.
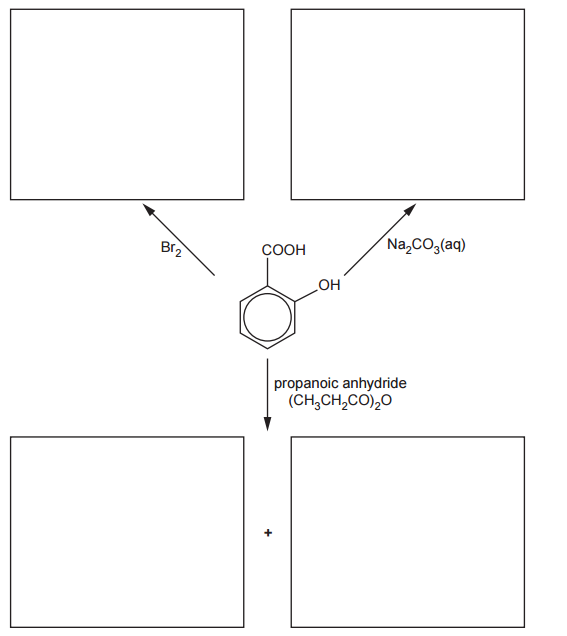
Reaction with Br2
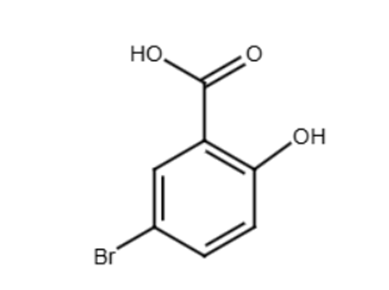
When salicylic acid reacts with bromine, bromine atoms are added to the benzene ring via a substitution reaction. The marking scheme for this question is very accommodating regarding the product structure. It explicitly allows for bromine substitution at any position on the ring and permits a product with up to four bromine atoms attached to the ring.
The specific structure shown in the mark scheme as an example is
Any logically defensible structure showing bromine substitution on the salicylic acid ring (with both the -OH and -COOH groups remaining, and up to four bromines) would be considered correct. The following structures would be also acceptable:
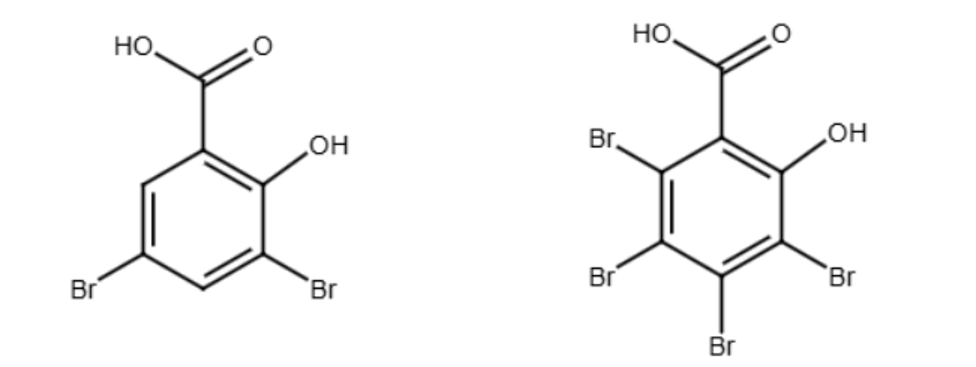
Reaction with Na2CO3(aq)
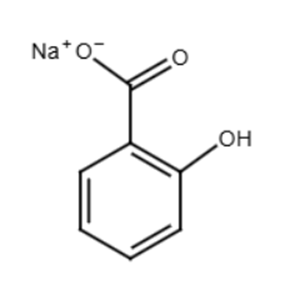
The reaction with sodium carbonate (Na2CO3) is a neutralization reaction. The reactivity is due to the carboxylic acid group (-COOH) within salicylic acid. Although salicylic acid also contains a phenolic hydroxyl group (-OH), this group is a significantly weaker acid compared to the carboxylic acid group, and sodium carbonate is not a strong enough base to effectively deprotonate the phenolic -OH group to a significant extent in this reaction.
2C6H4(OH)COOH(aq) + Na2CO3(s) → 2C6H4(OH)COONa(aq) + H2O(l) + CO2(g)
The product obtained is:
Reaction with propanoic anhydride (CH3CH2CO)2O
When salicylic acid reacts with propanoic anhydride, we get two organic products: an ester and an acid.
The primary product is the ester, 2-(propionyloxy)benzoic acid. This is formed when the propionyl group from the anhydride attaches to the -OH group of salicylic acid:
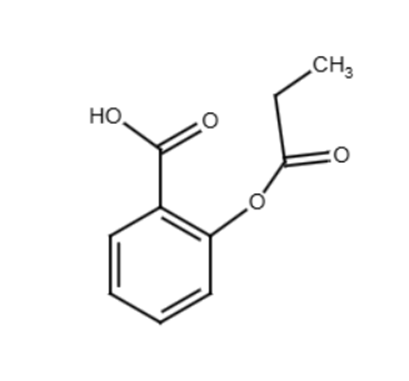
However, exam questions often ask for a second organic product. It’s easy to overthink this and look for another complex structure. But the second organic product formed in this reaction is simply propanoic acid, which is the other part of the anhydride molecule.
CH3-CH2-COOH
The answer from the mark scheme is:
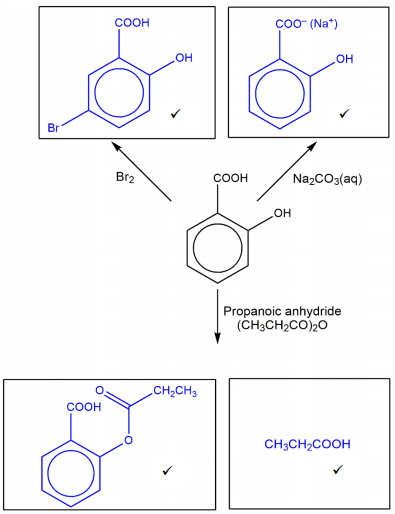
[4]
(b)
PAS, shown below, is an antibiotic used to treat several diseases including tuberculosis (TB)
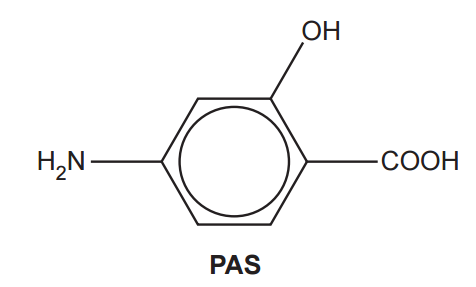
(i)
A student predicts that PAS could polymerise to form a polymer containing both ester and amide linkages.
Draw a section of this polymer.
The section should contain one amide and one ester linkage, which should be displayed.
Each PAS molecule has an amine, hydroxyl and carboxyl groups:
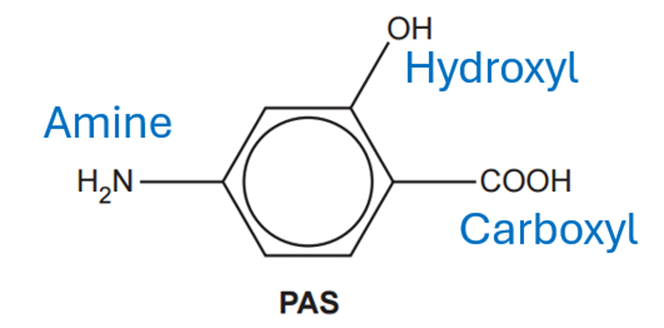
With these groups we need to create an amine group and an ester group:
As we are going to create 2 new links, we are going to use 3 benzene groups. Let’s start with the creation of an amine group between the amine and the carboxyl group:
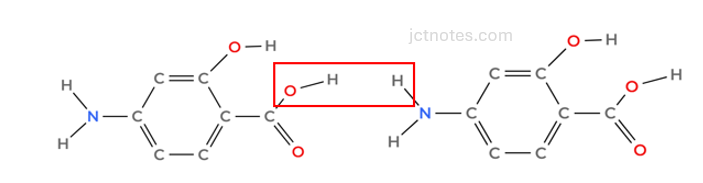
to create the amine linkage
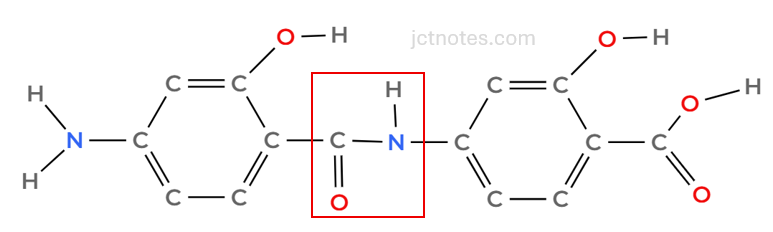
Now, we need to add another PAS molecule, creating a link between the -OH and the -COOH groups. We have a few possibilities; I choose the following (your choice will be also good as long as you add another benzene linking OH and COOH groups):
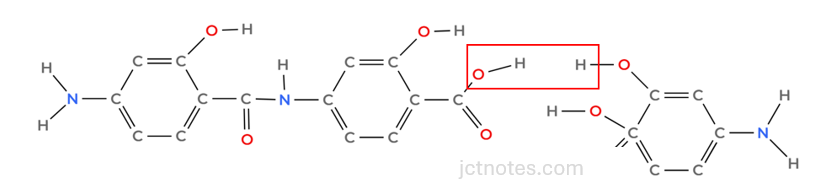
The ester group formed is:
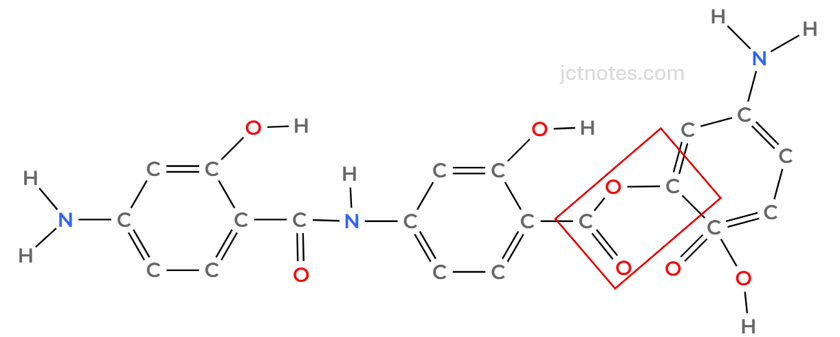
Finally, we add, at least, 1 end bond. I select 2, ensuring that the end bonds are different:
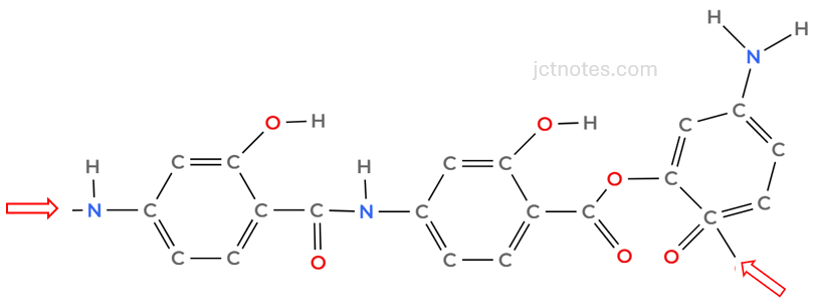
[3]
(ii)
For the treatment of TB, the maximum daily dosage of PAS that should be prescribed is 300mg per kg of body mass.
A child weighs 20.0 kg.
Calculate the number of PAS molecules in the maximum daily dosage of PAS for this child.
The maximum dosage for a 20.0 kg child is 300 × 20 = 6,000 mg (= 6 g) of PAS.
As the molecular mass of the PAS (C7H7NO3) is 12.0×7 + 1.0×7 + 14.0×1 + 16.0×3 = 153.0 g/mol, 6 g of PAS is
$$ \small \mathrm{6\,g \times \frac{1\,mol}{153.0\,g} = 3.9216 \times 10^{-2}\,mol} $$
Multiplying by Avogadro number:
3.9216×10-2 × 6.02×1023 = 2.36×1022 molecules of PAS
number of PAS molecules = ………………2.36×1022………………………………………
[3]
21
This question is about α-amino acids.
(a)
The general formula of an α-amino acid is RCH(NH2)COOH.
Most α-amino acids show optical isomerism.
Explain the term optical isomerism.
Stereoisomers are molecules that have the same structural formula (the same atoms are connected in the same order) but differ in the spatial arrangement of their atoms. In the case of OCR, it is referred to as in terms of non-superimposable mirror images about a chiral centre.
[1]
(b)
The α-amino acid valine has the R group of –CH(CH3)2.
(i)
What is the systematic name of valine?
The best approach for this question is to draw the formula of the compound, select the longest chain and start numbering the carbons from the carboxylic group:
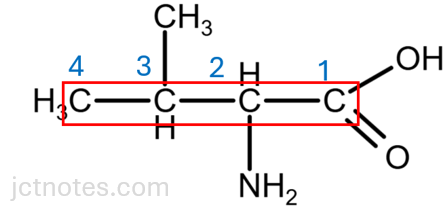
The systematic name of the valine is 2-amino-3-methylbutanoic acid.
[1]
(ii)
Draw diagrams to show 3D structures of the optical isomers of valine.
To draw optical isomers, first locate the chiral centre (a carbon atom bonded to four different groups). In the given structure, carbon 2 is the chiral centre. Next, visualize this carbon at the centre of a tetrahedron, with each of its four different groups positioned at the vertices. To draw the optical isomer, simply create a mirror image of this tetrahedral structure.
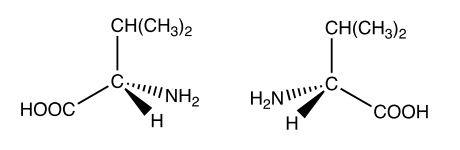
[2]
(c)
Three α-amino acids can react together to form compound E, shown below.
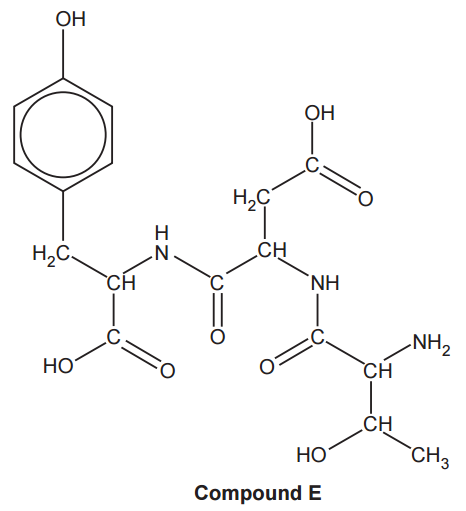
(i)
How many optical isomers are possible for compound E?
The number of optical isomers is given by the number of chiral carbon atoms (carbon atoms bonded to four different groups). Each carbon is going to give 2 possible optical isomers. If the number of chiral carbon atoms is n, the number of possible optical isomers is 2n. In compound E we have 4 chiral carbon atoms:
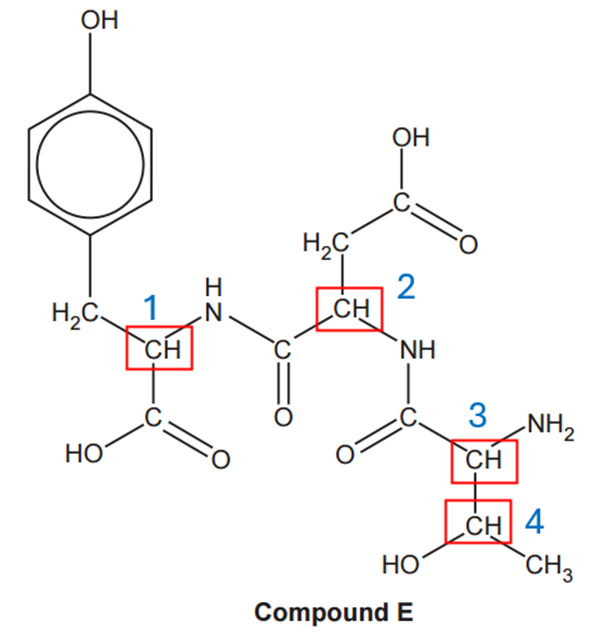
Therefore, the possible number of optical isomers is 24 = 16.
[1]
(ii)
A student hydrolyses compound E with dilute hydrochloric acid, HCl(aq).
Draw the structures of the organic products formed by this hydrolysis.
The reactions of the different amino acids can be explained as the reaction between the amino and the carboxylic group, producing an amide group and water:
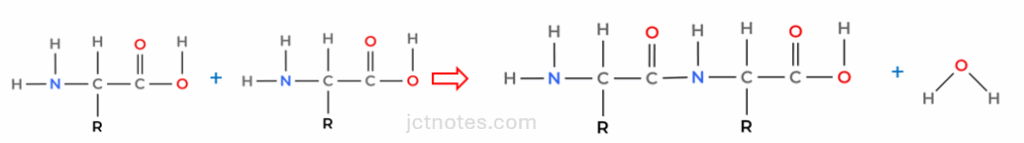
Hydrolysis (rupture with water) is the reverse process. We identify the amide group (-CO-NH-) and reverse the reaction. To ensure you get full marks, bear in mind that hydrolysis is performed in an acidic medium, therefore we should protonate the ammonia (NH4+).
The have 2 amide groups in compound E:
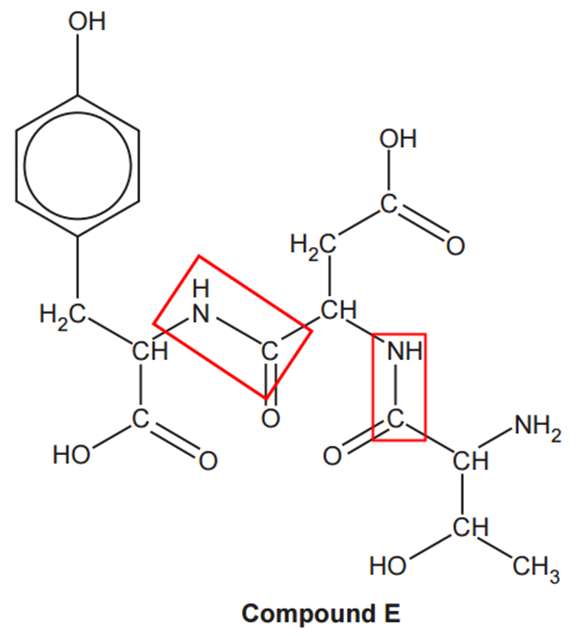
Therefore, we have 3 alpha amino acids. To avoid errors during drawing and easing the marking (it will work in your favour) draw the amino acids with the same orientation than the exam:
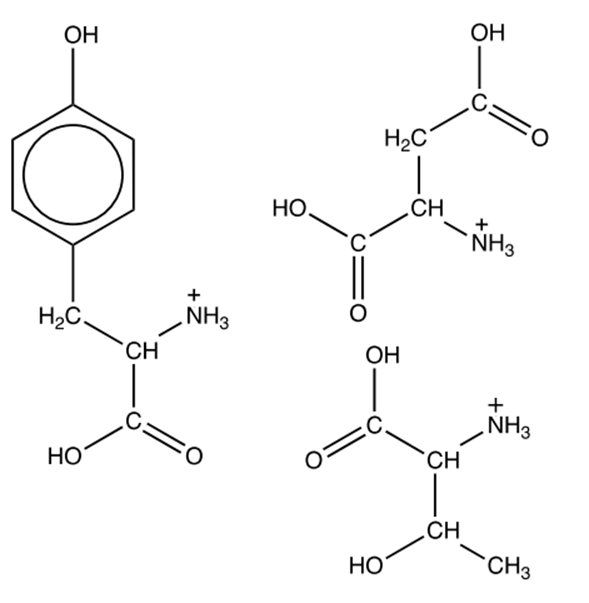
[4]
22
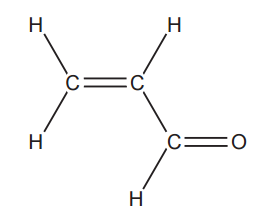
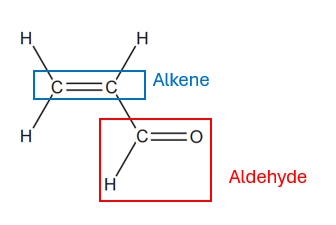
This question is about reactions of acrolein, H2C=CHCHO.
(a)
Acrolein reacts with sodium cyanide in acidic conditions, NaCN(aq)/H+(aq)
(i)
Outline the reaction mechanism for this reaction, showing the intermediate and the organic product.
The structure of acrolein has been provided.
Include curly arrows and relevant dipoles.
At A-level, we should treat this as a nucleophilic addition to an aldehyde. The OCR A Level Chemistry A – Topic Exploration Pack – Reaction pathways provides an excellent summary. While conjugate addition (or Michael addition) is a valid chemical reaction here and accepted on the mark scheme, it’s not usually on the A-level syllabus. To play it safe and guarantee marks in the exam, stick to the topics taught in the A level curriculum. However, we will see both reactions here.
In the nucleophilic addition to an aldehyde, the cyanide ion (CN–) acts as a nucleophile, attacking the electrophilic carbon of the aldehyde’s carbonyl group. This carbon has a partial positive charge because the C=O pi electrons are polarized towards the oxygen, which consequently gains a partial negative charge.
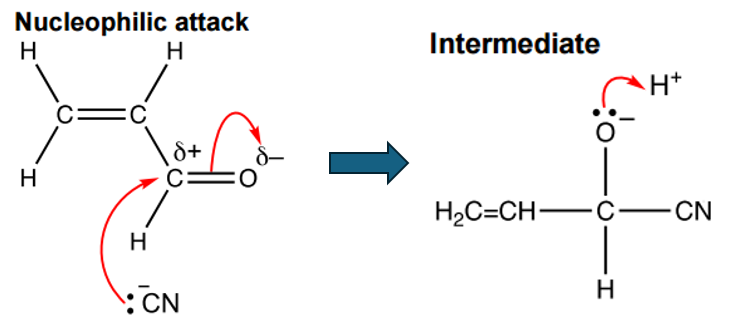
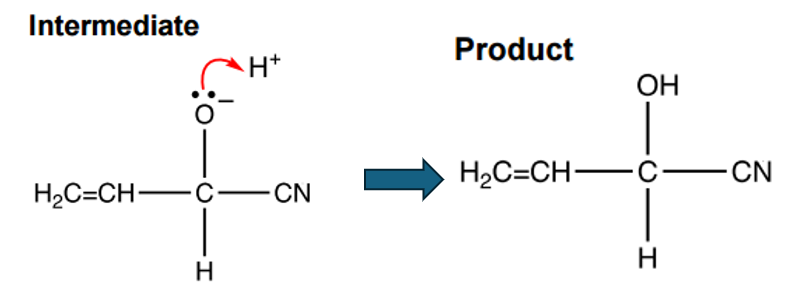
The negatively charged intermediate is highly reactive and quickly reacts with an H+ ion:
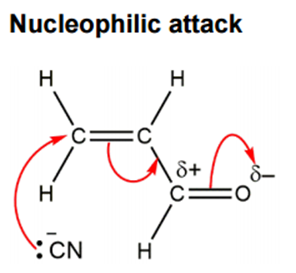
Conjugate addition (or Michael addition): acrolein, is known as an α,β-unsaturated carbonyl compound (an organic molecule with a carbonyl group (C=O) and a carbon-carbon double bond (C=C) directly connected to each other, separated by a single carbon atom) and is also an excellent Michael acceptor. As mentioned above, this is not covered in A level syllabus. In this case, the cyanide nucleophile attacks the β-carbon (the carbon furthest from the carbonyl, which is also part of the alkene).
The π-electrons of the C=C bond shift, and then the π-electrons of the C=O bond also shift onto the oxygen, forming an enolate intermediate. From the marking scheme, we get:
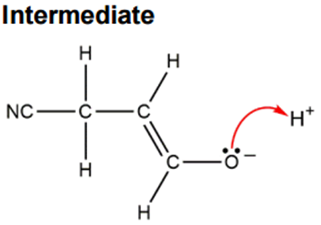
However, I believe that a better alternative would be:
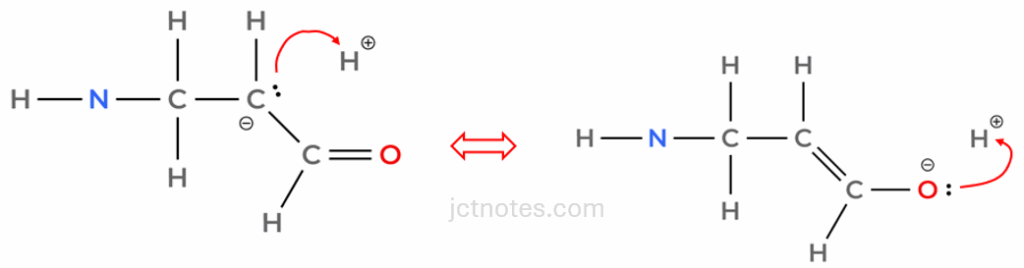
The enolate is then protonated by H+ (from the acidic medium) to give the saturated nitrile product.
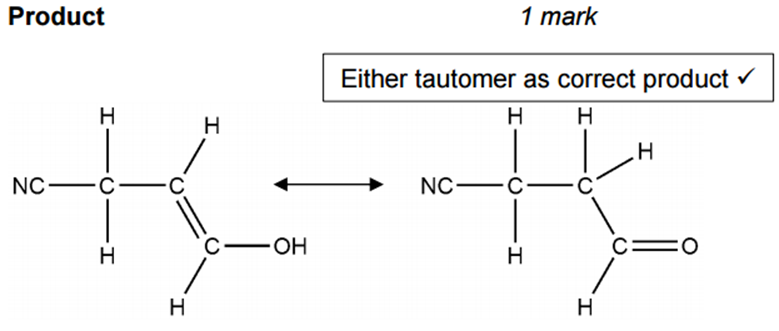
[4]
(ii)
Name this type of mechanism.
Given that a nucleophilic species has undergone an addition reaction with the initial product, this mechanism is classified as nucleophilic addition.
[1]
(b)
Complete the flowchart by filling in each box.
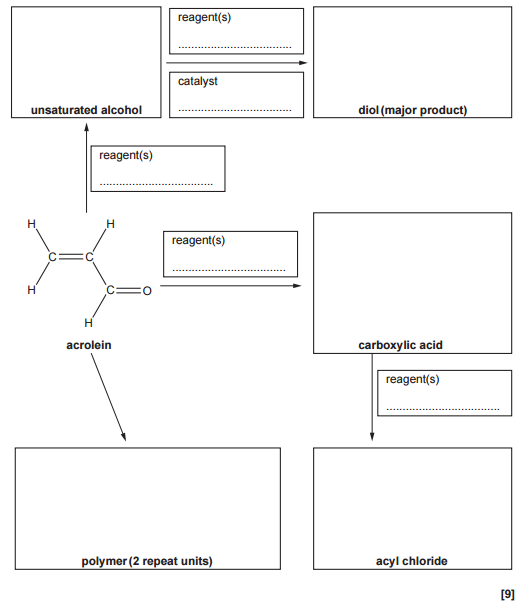
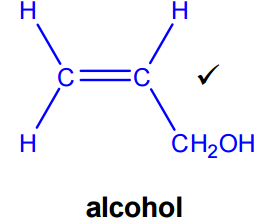
Formation of the unsaturated alcohol
To convert acrolein into an alcohol, there are two possible reactions to consider:
- Reduction of the aldehyde group
- Hydration of the alkene
Since the first product shown is an unsaturated alcohol, the C=C double bond must remain unchanged. This means the reaction is reduction of the aldehyde group only, not hydration of the alkene.
- Reduction of the aldehyde group in acrolein produces prop-2-en-1-ol (allyl alcohol).
- The reagent used is NaBH₄ (sodium borohydride).
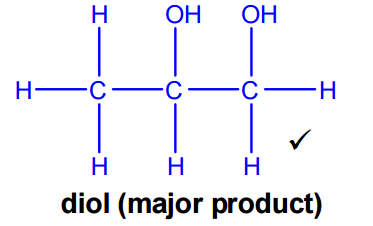
Formation of the diol (major product)
To form the diol, the alkene group in prop-2-en-1-ol undergoes hydration.
Because the question asks for the major product, Markovnikov’s rule must be applied:
When an unsymmetrical alkene reacts with an unsymmetrical reagent, the hydrogen atom adds to the carbon of the C=C bond that already has the most hydrogen atoms.
Applying this rule gives propane-1,2-diol (propylene glycol) as the major product.
- Reagent: water (steam)
- Catalyst: acidic conditions (H⁺, typically H₂SO₄ or H₃PO₄)
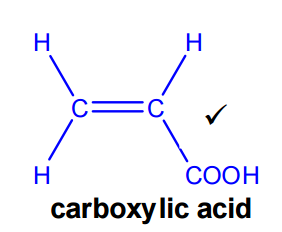
Formation of the carboxylic acid
Acrolein can be converted into a carboxylic acid by oxidation of the aldehyde group. The product formed is prop-2-enoic acid (acrylic acid).
- Reagents: acidified dichromate(VI). Acceptable answers include: K₂Cr₂O₇ / H₂SO₄ or Cr₂O₇²⁻ / H⁺
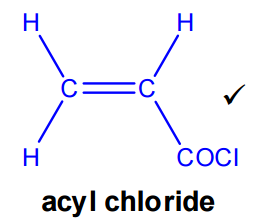
Formation of the acyl chloride
The carboxylic acid is converted into an acyl chloride by reaction with thionyl chloride (SOCl₂). Product: acryloyl chloride (2-propenoyl chloride)
Formation of the polymer
Acrolein can form a polymer via addition polymerisation using the C=C bond.
When drawing the polymer:
- Break the C=C double bond
- Join repeating units together
- Show two repeat units as required
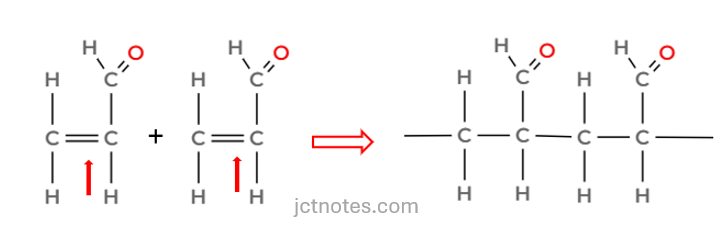
All together:
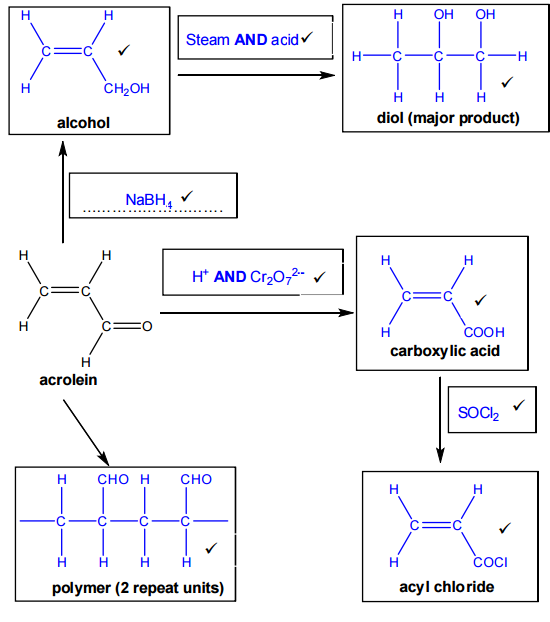
23*
An unknown organic compound is analysed.
The results are shown below.
Addition of 2,4-DNP
No visible change
Elemental analysis by mass
C, 66.63%; H, 11.18%; O, 22.19%
Mass spectrum
Molecular ion peak at m/ z = 144.0
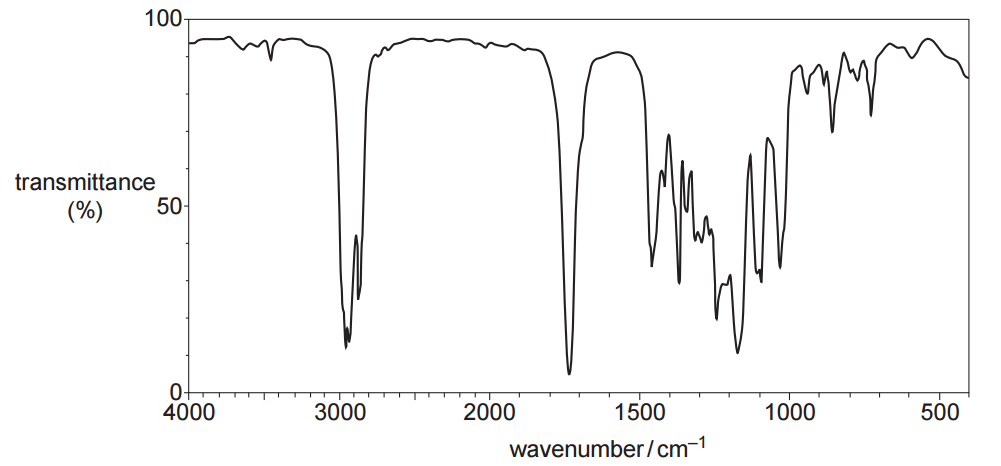
IR spectrum
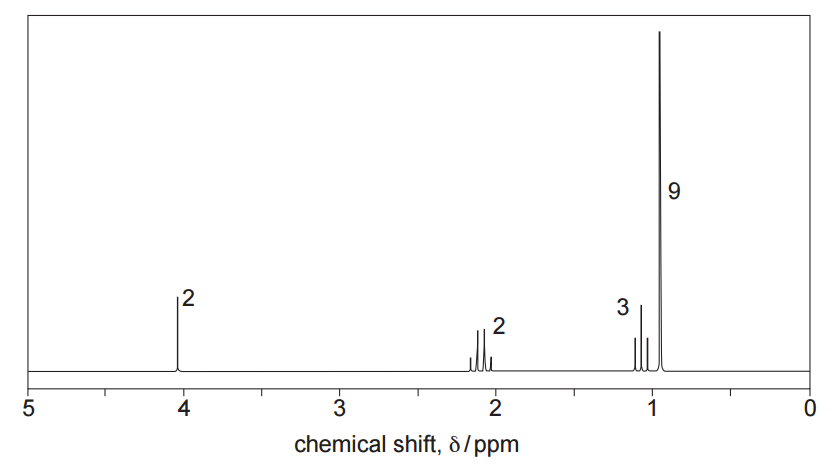
Proton NMR spectrum
The numbers by each peak are the relative peak areas.
Use the information to identify the organic compound.
Show all your reasoning
From the elemental analysis by mass, we can calculate the empirical formula:
| Atom | Atomic mass | Mass (or %) | Number of moles (mass/atomic mass) | Number of moles/lowest number of moles |
|---|---|---|---|---|
| C | 12 | 66.63 | 66.63÷12 =5.55 | 5.55÷1.38=4 |
| H | 1 | 11.18 | 11.18÷1=11.18 | 11.18÷1.38=8 |
| O | 16 | 22.19 | 22.19÷16=1.38 | 1.38÷1.38=1 |
The empirical formula is C4H8O.
The mass spectrum shows a molecular ion peak at m/z = 144. The relative formula mass of the empirical unit is:
(4 × 12) + (8 × 1) + (1 × 16) = 72
Hence:
72n = 144
n = 2
The molecular formula is C₈H₁₆O₂.
IR spectrum analysis
From the IR spectrum, the following key absorptions can be identified:
- A peak at approximately 3000 cm⁻¹, characteristic of C–H stretching in alkyl groups.
- A strong peak at approximately 1750 cm⁻¹, indicating the presence of a C=O bond.
- Peaks below 1,500 cm-1 are considered fingerprints of the compound.
Using this information and the additional data provided:
- Aldehydes and ketones can be excluded because the compound does not react with 2,4-DNP. If an aldehyde or ketone were present, an orange precipitate would form.
- Carboxylic acids can be excluded as they show a very broad O–H absorption between 2500–3000 cm⁻¹, which is not observed here.
- Amides and acyl chlorides can be excluded because the compound contains only carbon, hydrogen and oxygen.
- Acid anhydrides can be excluded because they require three oxygen atoms, whereas the molecular formula contains only two.
Therefore, the C=O absorption at ~1750 cm⁻¹ is consistent with an ester functional group.
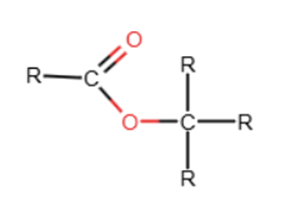
Proton NMR analysis
The proton NMR spectrum shows the following signals:
●
A singlet at 0.9 ppm integrating to 9 H, indicating three equivalent methyl groups attached to a carbon with no hydrogens, i.e. a tert-butyl group, C(CH₃)₃.
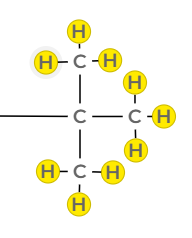
●
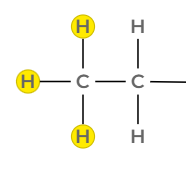
A triplet at 1.1 ppm integrating to 3 H, consistent with a methyl group adjacent to a CH₂ group (–CH₃CH₂–).
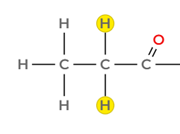
- A quartet between 2.0 and 2.3 ppm integrating to 2 H, consistent with a –CH₂– group adjacent to a carbonyl group (–CH₂–CO–). The quartet splitting confirms coupling with three neighbouring protons, matching the methyl group at 1.1 ppm.
- A singlet at 4.1 ppm integrating to 2H indicates a CH2 group linked by a single bond to an oxygen and to another carbon with no hydrogens
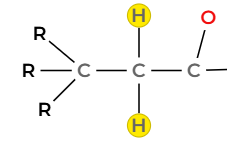
Conclusion
The molecular formula is C₈H₁₆O₂, and the spectroscopic data indicate the presence of an ester containing a tert-butyl group and an ethanoate fragment.
Combining all the evidence, the compound is identified as tert-butyl ethanoate (1,1-dimethylethyl ethanoate)
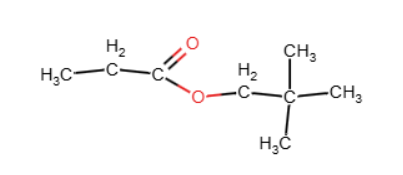
.
[6]
END OF QUESTION PAPER
Link to Section A.
Link to Section B. Questions 16 to 19.
