Link to Section B. Questions 16 to 19.
Link to Section B Questions 20 to 23.
Section A
1
Which compound is used for proton exchange in NMR spectroscopy?
A
CCl4
B
CDCl3
C
D2O
D
Si(CH3)4
In NMR spectroscopy, proton exchange refers to the process where labile hydrogen atoms (such as those in -OH, -NH, and -COOH groups) swap with deuterium from the solvent. This exchange helps identify and confirm the presence of these functional groups.
- Option A: CCl₄ (Carbon Tetrachloride): Used as a solvent in ¹H NMR because it lacks hydrogen atoms, preventing interference. However, it does not facilitate proton exchange.
- Option B: CDCl₃ (Deuterated Chloroform): A common NMR solvent that contains deuterium instead of hydrogen, reducing solvent signal interference. It does not promote proton exchange.
- Option C: D₂O (Deuterium Oxide): It enables proton exchange, replacing labile protons with deuterium, causing their signals to disappear from the ¹H NMR spectrum. This is the correct answer.
- Option D: Si(CH₃)₄ (Tetramethylsilane, TMS): Used as a reference compound in NMR, providing a sharp peak at 0 ppm for calibration. It does not participate in proton exchange.
Then, the answer is D₂O, (C).
Your answer: C
[1]
2
Which compound reacts with 2,4-dinitrophenylhydrazine but does not react with Tollens’ reagent?
A
C₆H₅COCOOH
B
C6H5CH(OH)CHO
C
CH3COCHO
D
CH3CH2CH(OH)CH3
The reaction with 2,4-dinitrophenylhydrazine (2,4-DNPH) confirms the presence of a carbonyl group, meaning the compound is either an aldehyde or a ketone. Since it does not react with Tollens’ reagent, it must be a ketone, as Tollens’ test is specific for aldehydes. Drawing the structure can help identify the ketone more easily.
Option A: C₆H₅COCOOH:
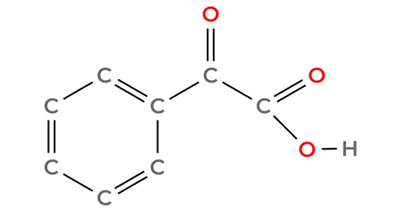
It has a ketone group, there are not aldehydes. This is the right answer.
Option B: C6H5CH(OH)CHO
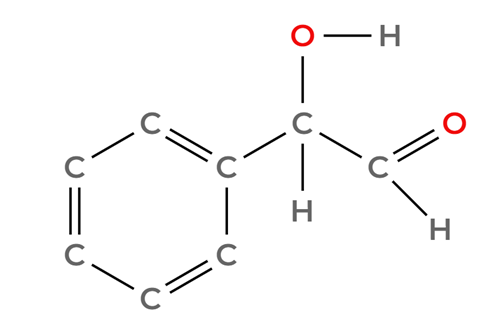
It is an aldehyde, it will react with Tollen’s reagent. We can discard it.
Option C: CH3COCHO
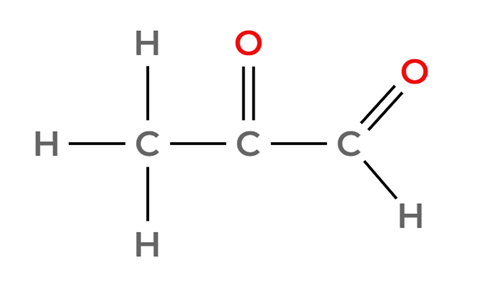
It is also an aldehyde, it will react with Tollen’s reagent. We can also discard it.
Option D: CH3CH2CH(OH)CH3
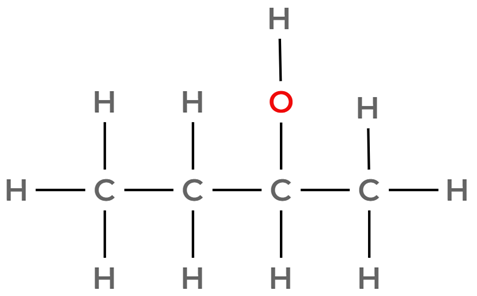
This is an alcohol, it will not react with 2,4-DNPH.
Your answer: A.
[1]
3
Propyne, CH3C≡CH, is a member of the alkynes homologous series with the C≡C functional group.
What is the general formula of the alkynes?
A
CnH2n–4
B
CnH2n–2
C
CnH2n
A
CnH2n+2
If you’re unsure whether the general formula for alkynes is CnH2n–2, the quickest way to determine the answer is through reverse engineering. Start by writing the molecular formula of the given compound, C₃H₄, and compare it to the answer choices. By matching the format, you confirm that the correct general formula is CnH2n–2, making option B the right answer.
Your answer: B
[1]
4
The structure of the painkiller paracetamol is shown below
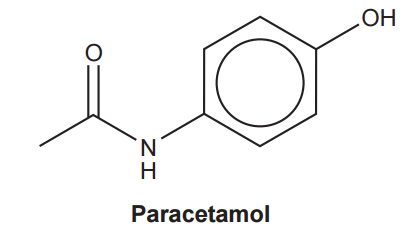
Which functional groups are present in paracetamol?
A
alcohol, amide
B
alcohol, arene, ketone, amine
C
phenol, amide
D
phenol, ketone, amine
In this type of problems, help to mark the functional groups. We have a phenol and a amide:
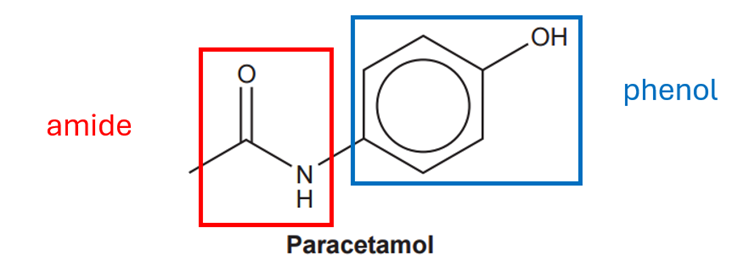
The answer is C.
Your answer: C
[1]
5
Oxymetazoline, shown below, is used as a decongestant in the treatment of colds.
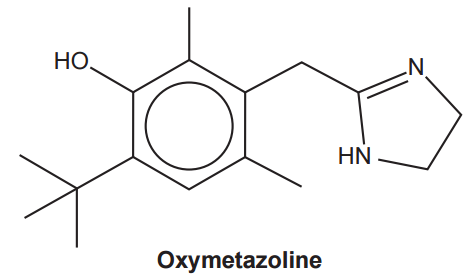
How many H atoms are in one molecule of oxymetazoline?
A
23
B
24
C
25
D
26
Labelling the number of H atoms in each carbon helps:
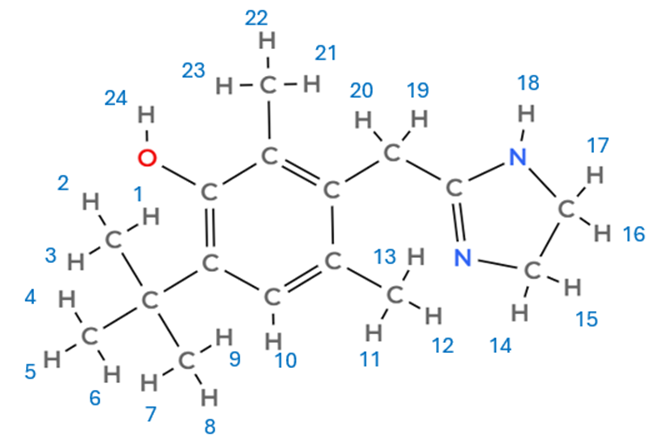
I reach a number of 24 atoms of H.
Your answer: B
[1]
6
Which statement supports the delocalised model of benzene and not the Kekulé model?
A
Sigma bonds overlap to form a π-system.
B
The carbon-carbon bond lengths are all the same.
C
The enthalpy change of hydrogenation is more exothermic than expected.
D
Benzene is more reactive than alkenes with bromine.
The delocalised model of benzene suggests that all carbon-carbon bonds are equal in length, somewhere between a single and double bond, due to the delocalisation of electrons across the ring. In contrast, the Kekulé model proposes alternating single and double bonds, which would result in different bond lengths. However, experimental evidence shows that benzene has uniform bond lengths, supporting the delocalised model over Kekulé’s structure..
Your answer: B
[1]
7
What is the systematic name for the compound below?
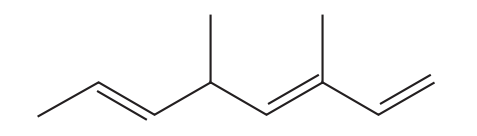
A
3,5-dimethylocta-1,3,6-triene
B
3,5-dimethylocta-2,5,7-triene
C
4,6-dimethylocta-1,3,6-triene
D
4,6-dimethylocta-2,5,7-triene
We begin by identifying the longest carbon chain, which consists of 8 carbon atoms:
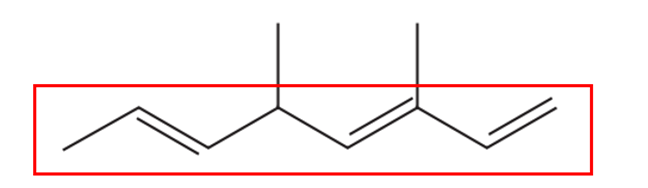
Next, we number the carbon atoms to ensure that substituents are assigned the lowest possible positions. If we number from the left, the lowest-positioned radical is 2-ene. However, if we number from the right, we get 1-ene, which takes priority according to naming conventions. Therefore, we start numbering from the right to correctly name the compound.
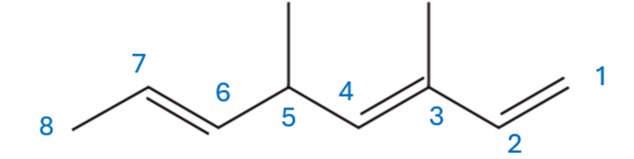
The molecule has methyl groups at positions 3 and 5 and double bonds at 1, 3, and 6. So, following the naming rules, the proper name is 3,5-dimethylocta-1,3,6-triene—”octa” for the 8 carbon chain and “triene” because of the three double bonds.
Your answer: A
[1]
8
For complete combustion, 0.100 mol of an alkane requires 22.8dm3 of O2, measured at RTP.
Which alkane has undergone complete combustion?
A
pentane
B
hexane
C
heptane
D
octane
We start by calculating the number of moles of O2 consumed. As 1 mol at RTP occupies 24 dm3, then, 22.8 dm3 is:
$$ \small \mathrm{ 22.8 \, dm^3 \times \frac{1 \, mol \, O_2}{24 \, dm^3} = 0.95 \, mol \, O_2 } $$
We then calculate the ratio of alkane to oxygen for each complete combustion:
Option A: pentane
C₅H₁₂ + 8 O₂ → 5 CO₂ + 6 H₂O
Option B: hexane
C₆H₁₄ + 9.5 O₂ → 6 CO₂ + 7 H₂O
Option C: Heptane
C₇H₁₆ + 11 O₂ → 7 CO₂ + 8 H₂O
Option D. Octane
C₈H₁₈ + 12.5 O₂ → 8 CO₂ + 9 H₂O
We can see that for the complete combustion of 0.1 mol of hexane we require 0.95 moles of O2, the answer is B.
Your answer: B
[1]
9
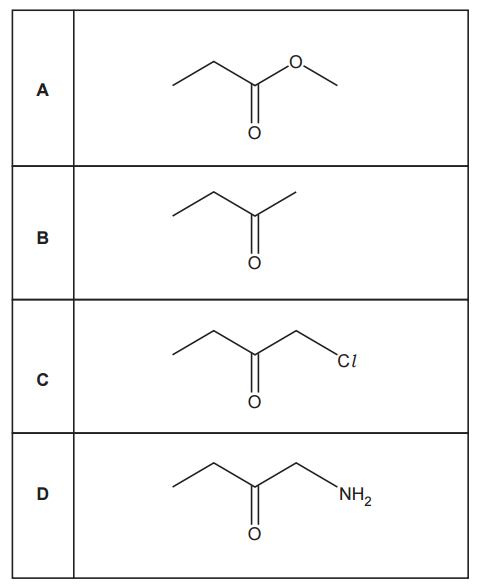
Which compound has the greatest number of peaks in its proton NMR spectrum?
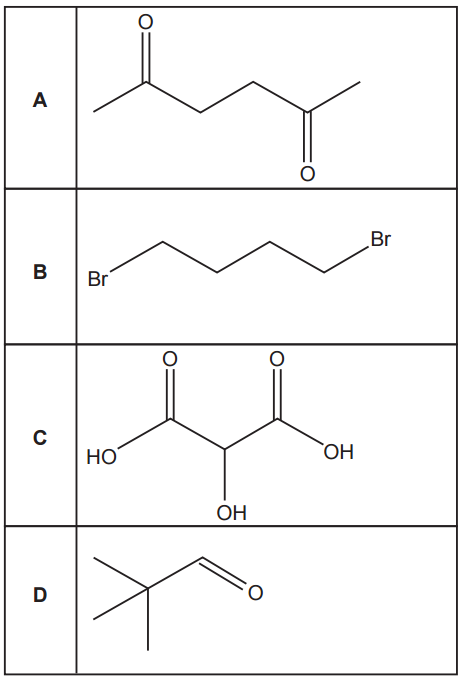
To determine which compound has the greatest number of peaks in its proton NMR spectrum, we need to identify unique proton environments (chemically and magnetically non-equivalent H atoms) and count the number of distinct signals.
Option A:
Symmetrical molecule.
- CH₃ (methyl) groups on both ends: equivalent → 1 environment
- CH₂ next to CO (carbonyl): both are equivalent → 1 environment
Total: 2 unique proton environments give 2 NMR peaks.
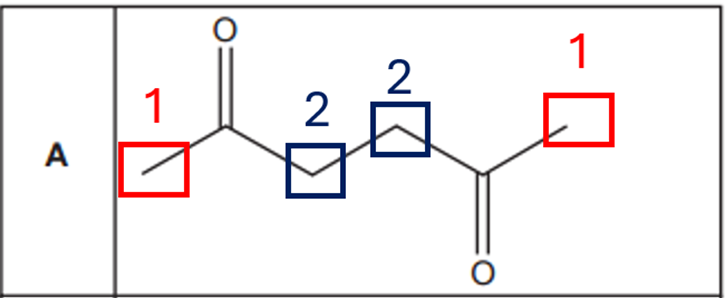
Option B:
Symmetrical molecule.
- CH₂ next to Br (terminal CH₂ groups): equivalent → 1 environment
- Internal CH₂ (middle two): also symmetrical → 1 environment
Total: 2 unique proton environments give 2 NMR peaks
.
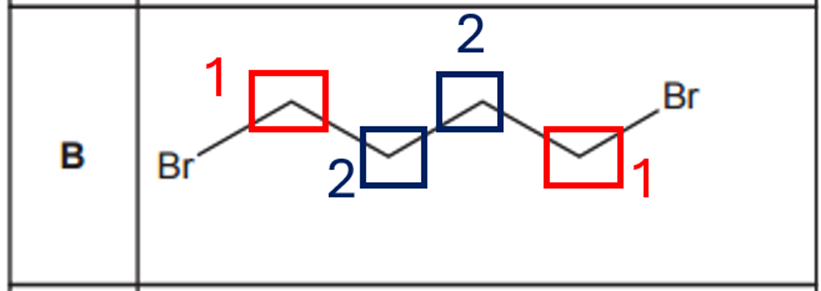
Option C:
Symmetrical molecule.
- 2 COOH hydrogens on both ends: 1 environment
- CH in the central C: 1 environment
- OH hydrogen in the central carbon: 1 environment
Total: 3 distinct proton environments give 3 NMR peaks
Option D.
- 3 CH₃ groups: equivalent due to symmetry → 1 environment
- OH hydrogen: 1 environment
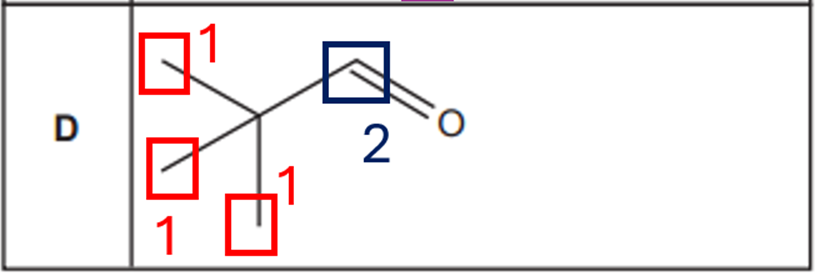
Total: 2 unique proton environments give 2 NMR peaks
Then, the option that has the greatest number of peaks in its proton NMR spectrum is option C with 3 peaks.
Your answer: C
[1]
10
Which ester is most likely to produce a mass spectrum with a fragment ion at m/z = 43?
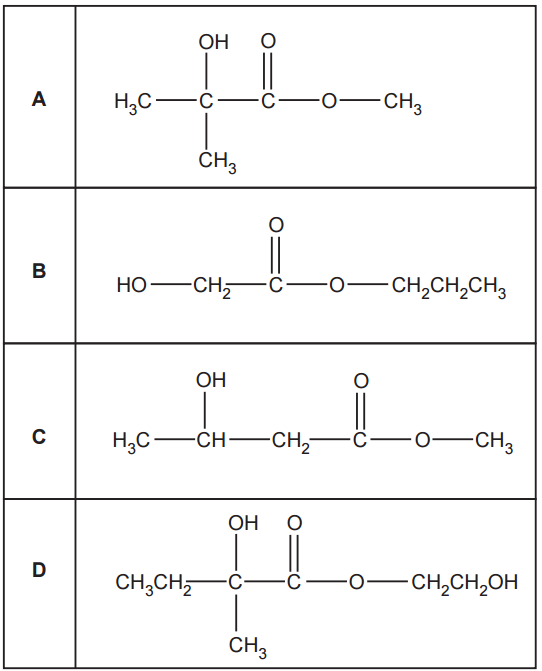
The esters given are combinations of H, C and O. The most probable ions that could give m/z = 43 are C3H7+ and C2OH3+. Looking at the given structures, we can easilty identify the propyl group in option B that gives a m/z = 43.
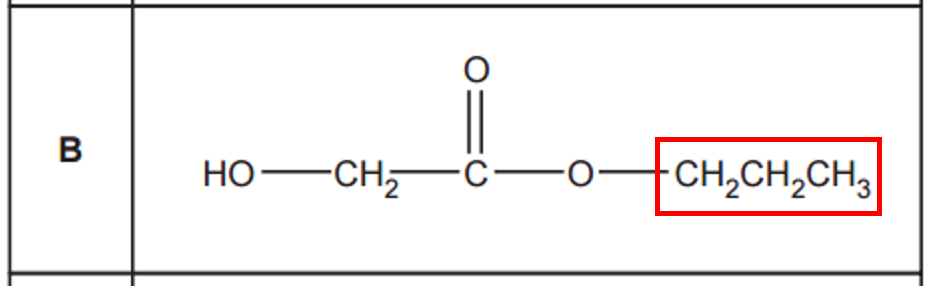
While other esters might, in theory, yield fragments of m/z=43 through more intricate rearrangements or multiple bond cleavages, the direct scission of the propyl group in Ester B represents a highly probable and characteristic fragmentation that would result in a prominent peak at m/z=43. This makes Ester B the most likely candidate among the options to produce this specific fragment ion.
Your answer: B
[1]
11
The infrared spectrum of an organic compound is shown below.
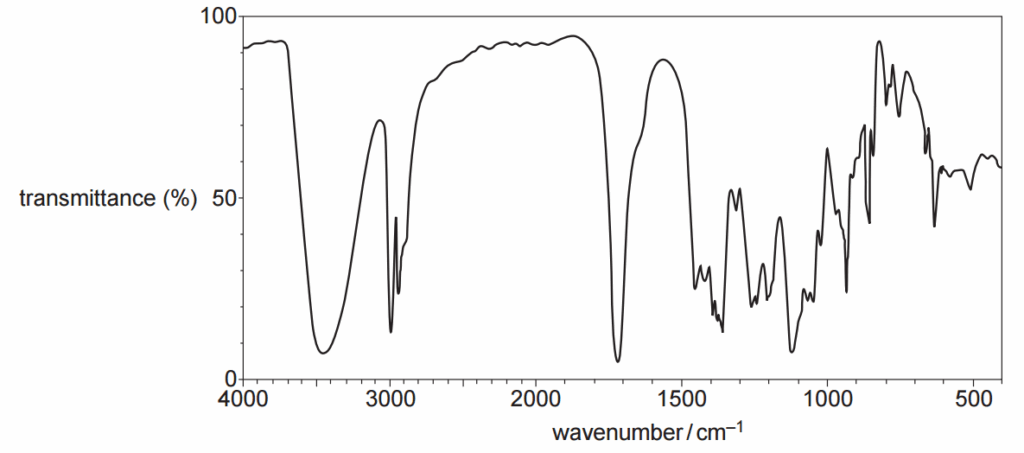
Which compound could have produced this spectrum?
From the data sheet of the spectrum, we can identify the following peaks:
- The peak at ~3500 cm-1 is an O-H bond from alcohols or phenols.
- The peak at ~3000 cm-1 is a C-H bond from alkyl groups, alkenes and arenes.
- The peak at ~1700 cm-1 is a C=O bond.
- The peaks below 1500 cm-1 are fingerprints.
We now check the different compounds given:
Option A has a carboxylic group. This is characterised by a broad peak between 2500 and 3300 cm-1. We have peaks in this region, but they are not broad, we discard option A,
Option B has C-H and C=O bonds, but it lacks O-H bonds, we discard option B.
Option C has O-H, C-H and C=O bonds. Option C could give the profile shown.
Option D has C-H and O-H bonds, but it lacks C=O bonds, we discard option D.
The only option that fit the profile is C.
Your answer: C
[1]
12
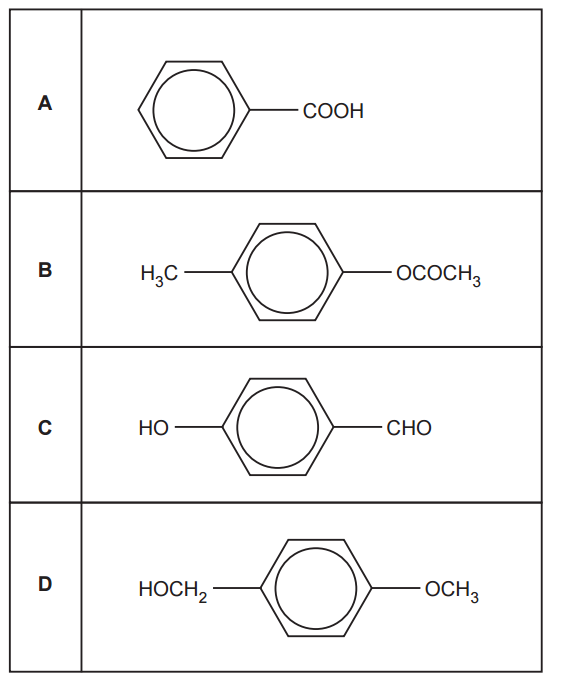
Which compound reacts with ethanoyl chloride?
Ethanoyl chloride, like other acyl chlorides, reacts with water, ammonia, primary amides, and alcohols (the link OCR A Level Chemistry A – Topic Exploration Pack – Reaction pathways is very helpful for this exam). Among the four given compounds, only option D falls into one of these reactive categories, as it is an amine.
Your answer: D
[1]
13
Which compound(s) is/are aliphatic?
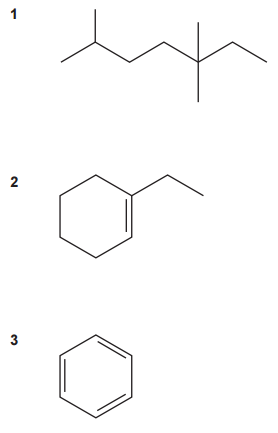
A
1, 2 and 3
B
Only 1 and 2
C
Only 2 and 3
D
Only 1
Aliphatic compounds are organic molecules that consist of carbon and hydrogen arranged in open chains or non-aromatic rings. They can be saturated (alkanes) or unsaturated (alkenes and alkynes) depending on the presence of single, double, or triple bonds. From the given structures:
Compound 1 is an open chain. It is an aliphatic compound.
Compound 2 is a non-aromatic ring. It is an aliphatic compound.
Compound 3 is an aromatic ring (cyclic structure with delocalized electrons). It is not an aliphatic compound.
Therefore, only compounds 1 and 2 are aliphatic compounds. The answer is B.
Your answer: B
[1]
14
Which compound(s) is/are hydrolysed by HCl(aq) to produce butanoic acid?
1
CH3CH2CH2COOCH3
2
CH3CH2CH2CH2CN
3
CH3CH2CH2CH2Cl
A
1, 2 and 3
B
Only 1 and 2
C
Only 2 and 3
D
Only 1
Butanoic acid has the formula CH3CH2CH2COOH.:
Compound 1. CH3CH2CH2COOCH3
This is an ester. Esters undergo acid-catalysed hydrolysis to produce a carboxylic acid and an alcohol. In this case, the reaction is
CH3CH2CH2COOCH3 + H2O + HCl(aq) → CH3CH2CH2COOH +CH3OH
Here, methyl butanoate hydrolyses to produce butanoic acid and methanol. So, 1 is correct.
Compound 2. CH3CH2CH2CH2CN
This is a nitrile. Nitriles undergo acid-catalysed hydrolysis to produce a carboxylic acid and an ammonium ion (which can then react with acid to form an ammonium salt). The carbon count is important here.
CH3CH2CH2CH2CN + H2O + HCl(aq) → CH3CH2CH2CH2COOH + NH4+
If we count the carbons in the starting nitrile, there are 5 carbons. Hydrolysis of this nitrile will produce pentanoic acid (CH3CH2CH2CH2COOH), not butanoic acid.
Therefore, 2 is incorrect as it produces pentanoic acid, not butanoic acid.
Compound 3. CH3CH2CH2CH2Cl
This is an alkyl halide. Alkyl halides can undergo hydrolysis (nucleophilic substitution) in the presence of water to form alcohols, especially with strong bases or sometimes under acidic conditions with heat. In the presence of HCl(aq), we can expect no reaction or very little reaction to produce some butan-1-ol, not butanoic acid:
CH3CH2CH2CH2Cl + H2O → CH3CH2CH2CH2OH + HCl
So, 3 is incorrect.
Therefore, only compound 1 produces butanoic acid. The answer is D.
Your answer: D
[1]
15
Which ion(s) contain(s) bond angles of approximately 120º?
1
CH3COO–
2
C6H5O–
3
(CH3)3C+
A
1, 2 and 3
B
Only 1 and 2
C
Only 2 and 3
D
Only 1
Ion 1. CH3COO– (Acetate ion):
- The carbon atom in the CH3 group is sp3 hybridized, leading to approximate bond angles of 109.5°.
- The carbon atom in the COO– group (the carboxylate group) is sp2 hybridized. Due to resonance, the two C-O bonds are equivalent and have partial double bond character. This central carbon has three electron domains (one C-C single bond and two C-O bonds, with the negative charge delocalized). This arrangement leads to a trigonal planar geometry around this carbon, with bond angles of approximately 120°.
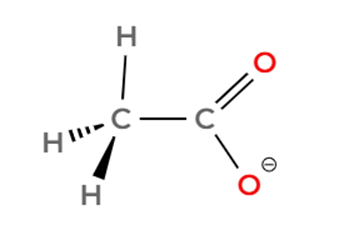
Ion 2. C6H5O– (Phenoxide ion):
- This ion consists of a benzene ring with an oxygen atom attached, which carries a negative charge.
- The carbon atoms within the benzene ring are sp2 hybridized, and the bond angles within the ring are approximately 120° (due to its trigonal planar geometry).
- The oxygen atom is bonded to one carbon atom of the benzene ring. While the C-O bond itself is not part of a 120-degree angle with other atoms directly bonded to the oxygen, the overall structure incorporates 120-degree angles within the aromatic ring. The angle at the oxygen atom linked to the phenyl group would be influenced by lone pairs on oxygen and resonance, but the key angles of 120 degrees are present in the aromatic ring.
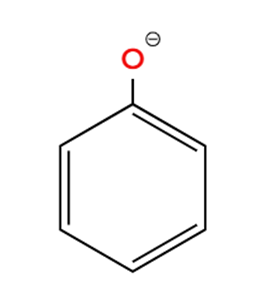
Ion 3. (CH3)3C+ (tert-Butyl carbocation):
- The central carbon atom in (CH3)3C+ is a carbocation, meaning it has only three groups attached (three methyl groups) and no lone pairs.
- This leads to sp2 hybridization and a trigonal planar geometry around the central carbon.
- Therefore, the C-C-C bond angles are approximately 120°.
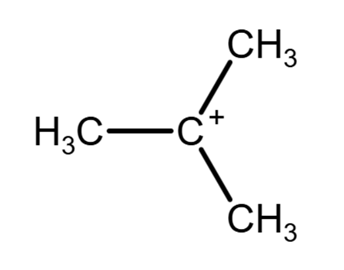
Therefore, ions 1, 2 and 3 contain bond angles of approximately 120º. The answer is A.
Your answer: A
[1]
Link to Section B. Questions 16 to 19.
Link to Section B Questions 20 to 23.
